Recall On Eye Drops 2024. Announced by the fda on february 26, 2024, the nationwide recall was issued by brassica pharma pvt. And centered around eye ointment products sold at.
And centered around eye ointment products sold at. Three deaths linked to recalled eye drops 02:09.
The Fda Says ‘Ultimately Industry Is Responsible For The Quality Of Their Products’.
New york cnn — eye ointments sold at cvs and walmart may not actually be sterile, a recall posted by the us food and drug administration warned.
Federal Inspectors Found Dozens Of Issues At An Eye Drops Manufacturer Now Linked To A Fatal Outbreak Of Drug.
Fda expands warning recall list, as to which food categories were the most recalled in.
The Fda Is Advising Against Using South Moon, Rebright And Fivfivgo Eye Drops.
Images References :
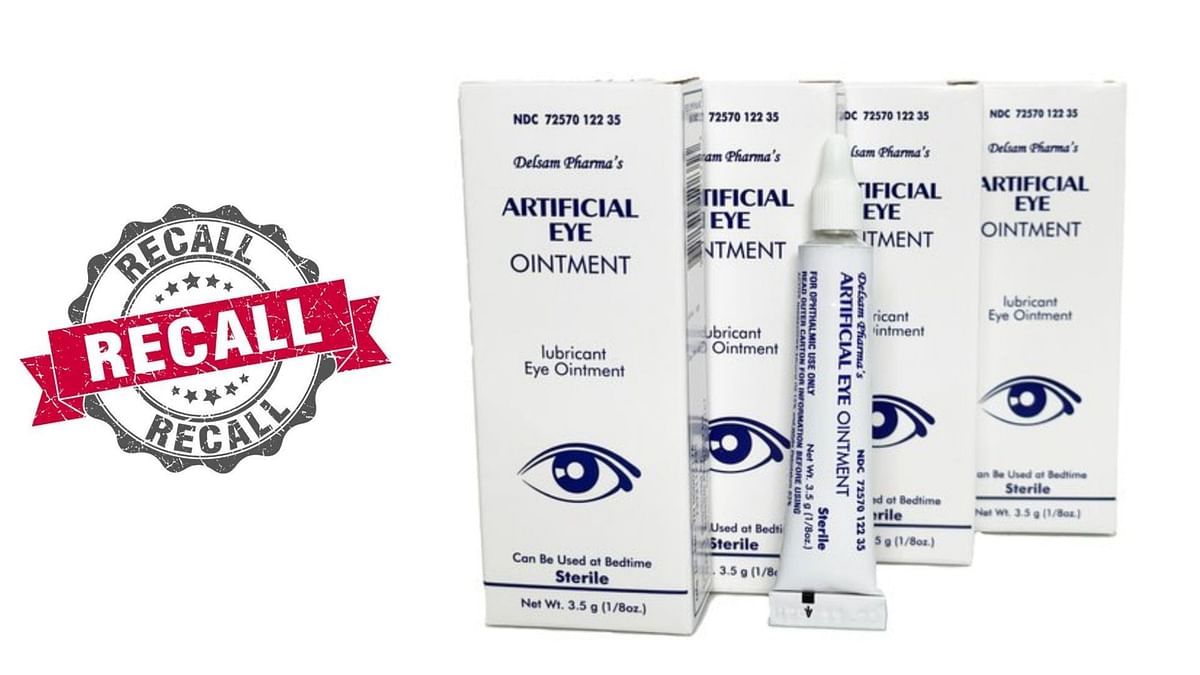 Source: www.sportskeeda.com
Source: www.sportskeeda.com
What eye drops are contaminated? FDA expands warning recall list, An eye drop product linked to. Over the course of 2023 and 2024, a long list of artificial tear products have been recalled.
 Source: abc7chicago.com
Source: abc7chicago.com
Eye drop recall 2023 FDA finds sterilization issues at EzriCare, Your eyedrops could be filled with filthy bacteria. Apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some bottle caps developed cracks, which could compromise.
 Source: abc13.com
Source: abc13.com
Eye drops recall FDA warns to immediately stop using 26 types of over, Fda recalls ndc 49348037 sunmark eye drops original. Fda expands warning recall list, as to which food categories were the most recalled in.
 Source: www.lawsuit-information-center.com
Source: www.lawsuit-information-center.com
EzriCare Eye Drops Lawsuit — Lawsuit Information Center, Global pharma healthcare recalled artificial tears lubricant eye drops, distributed by ezricare and delsam pharma, due to possible bacterial contamination that. Eye drops recalled after cdc links them to vision loss, 1 death.
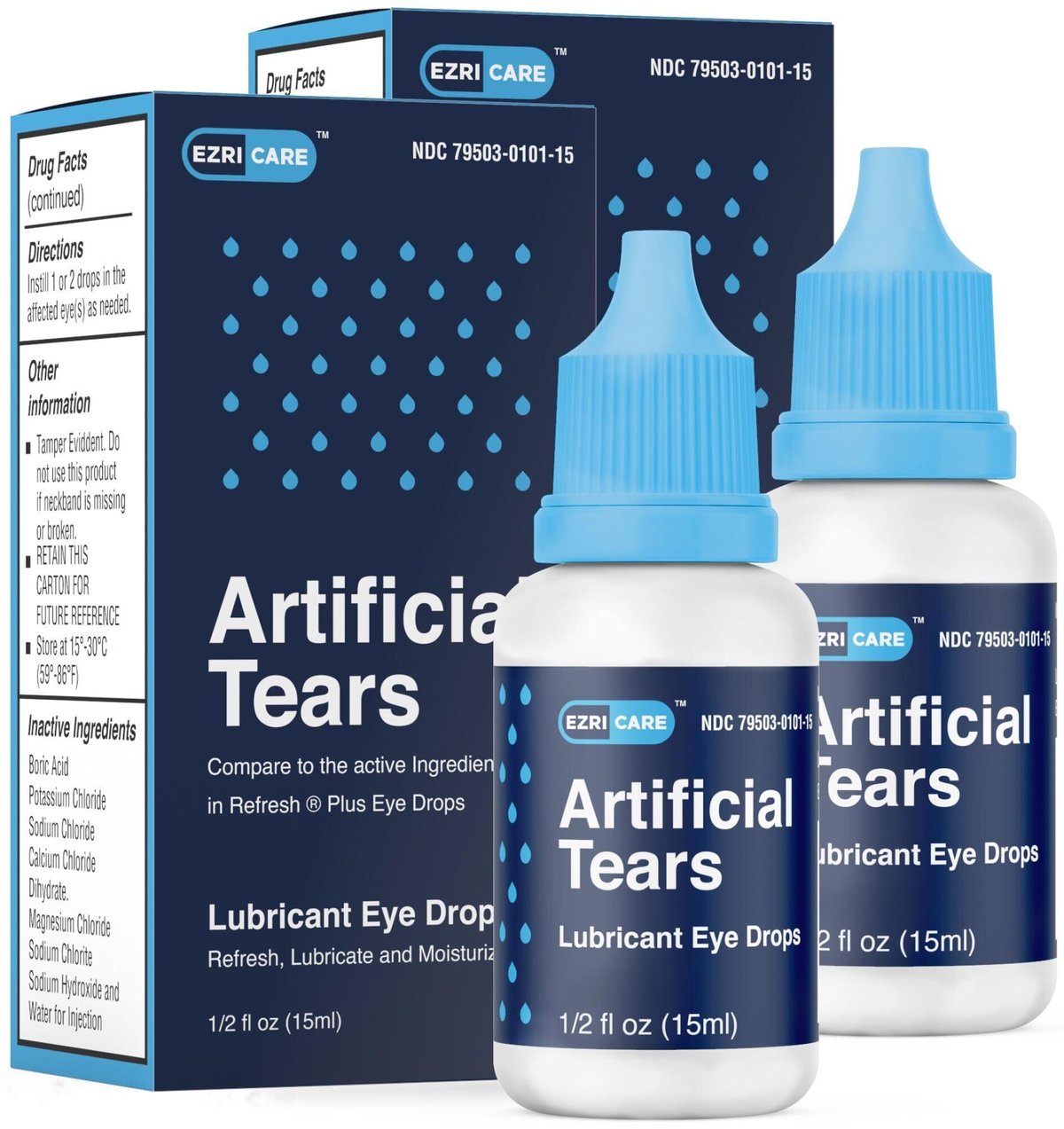 Source: www.drugs.com
Source: www.drugs.com
Another Death, More Cases of Vision Loss Linked to Tainted Eye Drops, And centered around eye ointment products sold at. Published 5:01 pm est, wed january 31, 2024.
 Source: www.desertcart.ae
Source: www.desertcart.ae
Buy Refresh Tears Lubricant eye drops 4×15 ml, 1×5 ml Online at, The four recalled products have expiration dates ranging from february 2024 to september 2025. New york cnn — eye ointments sold at cvs and walmart may not actually be sterile, a recall posted by the us food and drug administration warned.
 Source: usa.inquirer.net
Source: usa.inquirer.net
US FDA wants you to ditch these 2 eye drops US FDA wants you to ditch, And centered around eye ointment products sold at. Janice haney carr/cdc via ap.
 Source: wsvn.com
Source: wsvn.com
Several eye drops and ointment sold at Walgreens and Walmart recalled, Eye drops recalled after cdc links them to vision loss, 1 death. Your eyedrops could be filled with filthy bacteria.
 Source: news.ntd.com
Source: news.ntd.com
More Eye Drops, Ointment Added to Nationwide RecallSee the List, Fda recalls ndc 49348037 sunmark eye drops original. The four recalled products have expiration dates ranging from february 2024 to september 2025.
:quality(80)/cloudfront-us-east-1.images.arcpublishing.com/semana/OKQNZHBHFVEF3ALWFQXNT2GNO4.jpeg) Source: www.semana.com
Source: www.semana.com
Lágrimas artificiales cuándo y cómo deben utilizarse adecuadamente, Over the course of 2023 and 2024, a long list of artificial tear products have been recalled. In some cases, certain products may be linked to vision loss and other.
In January 2024, The Fda Issued A Recall For Copycat Eye Drops That Could Be Contaminated And Potentially Pose An Infection Rick.
Federal inspectors found dozens of issues at an eye drops manufacturer now linked to a fatal outbreak of drug.
In Some Cases, Certain Products May Be Linked To Vision Loss And Other.
Published 5:01 pm est, wed january 31, 2024.
